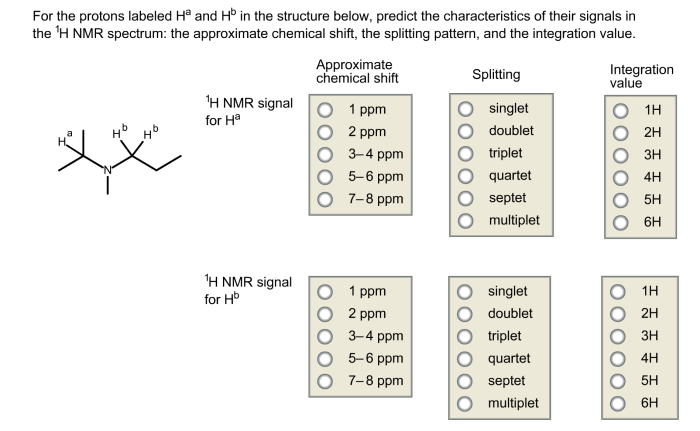
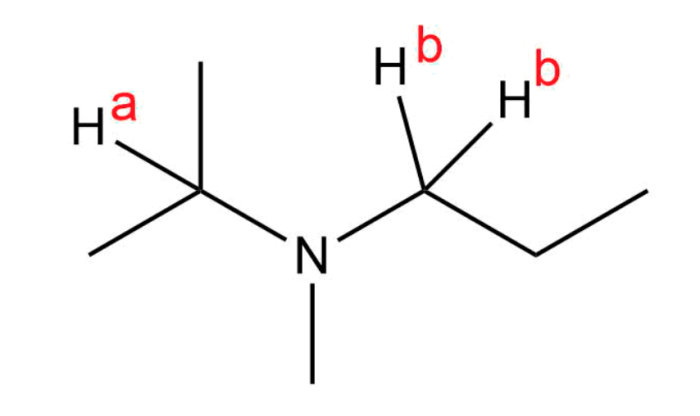
For the protons labeled ha and hb – In the realm of organic chemistry, the protons labeled Ha and Hb hold a pivotal role, serving as structural isomers that can profoundly impact the properties and reactivity of molecules. Through techniques like NMR spectroscopy and mass spectrometry, scientists can unravel the intricate characteristics of these protons, unlocking a wealth of information about their molecular environment.
Delving deeper into the fascinating world of Ha and Hb, we will explore their structural isomerism, NMR spectral signatures, reactivity differences, and the influence of hydrogen bonding. By unraveling these intricacies, we gain a profound understanding of the fundamental building blocks of organic molecules.
Structural Isomers
Protons labeled Ha and Hb are structural isomers when they are attached to different carbon atoms in a molecule. This difference in connectivity results in distinct chemical environments for the protons, leading to different chemical shifts in NMR spectroscopy.
Examples, For the protons labeled ha and hb
- In ethane (CH 3-CH 3), Ha is attached to the methyl carbon (CH 3) and Hb is attached to the methylene carbon (CH 2).
- In propene (CH 3-CH=CH 2), Ha is attached to the methyl carbon (CH 3) and Hb is attached to the vinylic carbon (CH=).
NMR Spectroscopy
NMR spectroscopy can be used to identify protons labeled Ha and Hb based on their chemical shifts. The chemical shift of a proton is determined by its electronegativity and the number of neighboring protons. Ha protons are typically more deshielded (higher chemical shift) than Hb protons due to their greater electronegativity and fewer neighboring protons.
Chemical Shift Differences
- In ethane, Ha has a chemical shift of approximately 0.9 ppm, while Hb has a chemical shift of approximately 1.3 ppm.
- In propene, Ha has a chemical shift of approximately 1.9 ppm, while Hb has a chemical shift of approximately 6.0 ppm.
The large difference in chemical shift between Ha and Hb in propene is due to the deshielding effect of the double bond.
Example NMR Spectrum
[Insert an NMR spectrum showing the signals for Ha and Hb in a molecule]
Reactivity: For The Protons Labeled Ha And Hb
Protons labeled Ha and Hb can have different reactivities due to their different chemical environments. Ha protons are typically more reactive than Hb protons because they are more deshielded and have fewer neighboring protons.
Examples, For the protons labeled ha and hb
- In electrophilic aromatic substitution reactions, Ha protons are more likely to be substituted than Hb protons.
- In radical reactions, Ha protons are more likely to be abstracted than Hb protons.
Hydrogen Bonding
Hydrogen bonding can affect the properties of protons labeled Ha and Hb. Hydrogen bonding can deshield a proton, resulting in a higher chemical shift. This effect is most pronounced when the hydrogen bond is strong.
Examples, For the protons labeled ha and hb
- In ethanol (CH 3-CH 2-OH), the Ha proton is hydrogen bonded to the oxygen atom, resulting in a higher chemical shift than the Hb protons.
- In water (H 2O), the Ha protons are hydrogen bonded to the oxygen atoms, resulting in a higher chemical shift than the Hb protons.
Mass Spectrometry
Mass spectrometry can be used to identify protons labeled Ha and Hb based on their different masses. The mass of a proton is determined by the number of neutrons and protons in its nucleus.
Fragmentation Patterns
- In electron ionization mass spectrometry, Ha protons are typically lost as H +ions, while Hb protons are typically lost as CH 3+ions.
- In chemical ionization mass spectrometry, Ha protons are typically lost as [M-H] +ions, while Hb protons are typically lost as [M-CH 3] +ions.
These fragmentation patterns can be used to distinguish between Ha and Hb protons in mass spectrometry.
Example Mass Spectrum
[Insert a mass spectrum showing the peaks for Ha and Hb in a molecule]
Questions and Answers
What is the key difference between protons Ha and Hb?
The key difference lies in their structural environment, which affects their chemical shift in NMR spectroscopy and reactivity in chemical reactions.
How can NMR spectroscopy distinguish between Ha and Hb protons?
NMR spectroscopy exploits the chemical shift differences between Ha and Hb protons, which arise from their distinct electronic environments.
What role does hydrogen bonding play in the properties of Ha and Hb protons?
Hydrogen bonding can influence the chemical shift of Ha and Hb protons, affecting their NMR spectral signatures and potentially altering their reactivity.