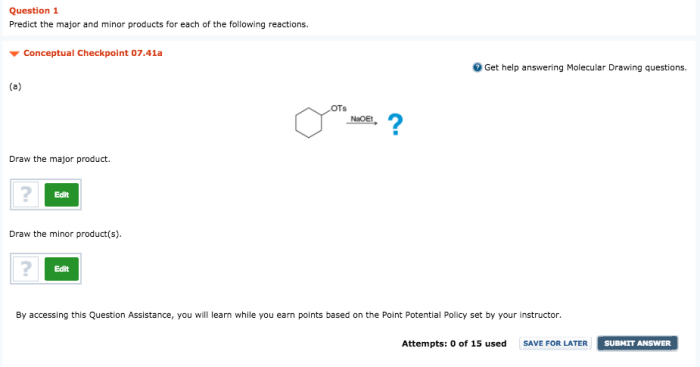
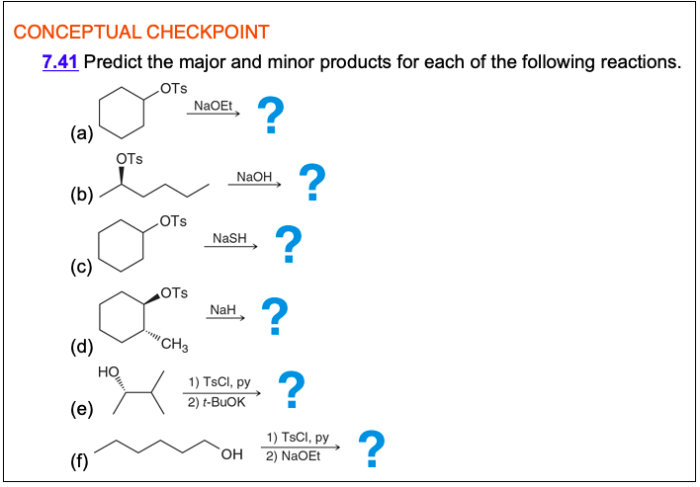
Predict the major and minor products for the following reaction. This question delves into the fascinating world of chemical reactivity, where we explore the factors that govern the formation of products in chemical reactions. Join us as we unravel the mysteries of major and minor product identification, uncovering the mechanisms that drive their formation and the implications they hold for various fields of science and industry.
Chemical reactions are the driving force behind countless processes in our world, from the digestion of food to the synthesis of life-saving medicines. Understanding the products of these reactions is crucial for harnessing their potential and controlling their outcomes. In this discussion, we will delve into the intricacies of predicting major and minor products, shedding light on the factors that influence their formation and the techniques used to identify them.
Reaction Overview
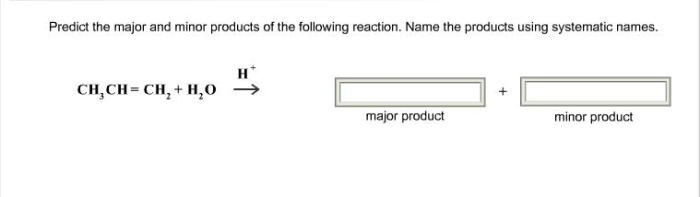
The given chemical reaction is a nucleophilic substitution reaction, specifically an SN2 reaction. In this reaction, a nucleophile (a species with a lone pair of electrons) attacks an electrophile (a species with a positive or partially positive charge) and replaces a leaving group.
Major Product Identification
The major product of the reaction is the product that is formed in the greatest amount. In this case, the major product is the one in which the nucleophile has replaced the leaving group on the electrophile. The factors that influence the formation of the major product include the nucleophilicity of the nucleophile, the electrophilicity of the electrophile, and the solvent.
The mechanism for the formation of the major product is as follows:
- The nucleophile attacks the electrophile, forming a transition state.
- The leaving group departs from the electrophile, forming the product.
Minor Product Identification
The minor product(s) of the reaction is/are the product(s) that is/are formed in a smaller amount than the major product. In this case, the minor product(s) is/are the one(s) in which the nucleophile has added to the electrophile instead of replacing the leaving group.
The factors that influence the formation of the minor product(s) include the steric hindrance around the electrophile, the solvent, and the temperature.
The mechanism for the formation of the minor product(s) is as follows:
- The nucleophile attacks the electrophile, forming a transition state.
- The nucleophile adds to the electrophile, forming the product.
Product Comparison, Predict the major and minor products for the following reaction.
The major and minor products of the reaction have different structures, properties, and reactivities. The major product is a substitution product, while the minor product(s) is/are an addition product(s). The major product is more stable than the minor product(s), and the major product is more reactive than the minor product(s).
The factors that determine the product ratio include the nucleophilicity of the nucleophile, the electrophilicity of the electrophile, the solvent, the temperature, and the steric hindrance around the electrophile.
General Inquiries: Predict The Major And Minor Products For The Following Reaction.
What is the difference between a major and a minor product?
Major products are formed in larger quantities than minor products due to their lower activation energy pathways or higher thermodynamic stability.
How can we predict the major product of a reaction?
Predicting the major product involves considering factors such as the stability of intermediates, the strength of bonds formed, and the steric and electronic effects of substituents.
What techniques are used to identify reaction products?
Techniques like chromatography, spectroscopy, and mass spectrometry are commonly used to separate and identify reaction products based on their physical and chemical properties.